The mitochondria: function, implications of dysfunction, and actions you can take to promote mitochondrial health
This comprehensive blog post represents the conclusion of Mitochondria May, an educational mini-series that was hosted on the Calorify Instagram.
If you’ve ever taken a biology class, you might remember that “the mitochondria is the powerhouse of the cell.” Here, we’ll explore what this actually means in terms of mitochondrial function and what that means for metabolic disease control.
But before we dive in, why is Calorify so interested in the mitochondria? Mitochondria play a key role in cellular respiration—turning food and O2 into CO2 and H2O to produce energy (ATP)—which is what Calorify measures!
Cellular Function
In order to better understand the mitochondria, let’s take a tour of the cell. First, there are two different types of cells: prokaryotes and eukaryotes. Prokaryotes are single-celled organisms like bacteria. They are missing organelles that eukaryotes have, and most distinctively, don’t have a membrane around their nucleus (rather, they have free-floating rings of DNA). Eukaryotes are much larger than prokaryotes, and do have a membrane around their nucleus. Animals, plants, and fungi are all made up of eukaryotic cells—so your cells are eukaryotic too.
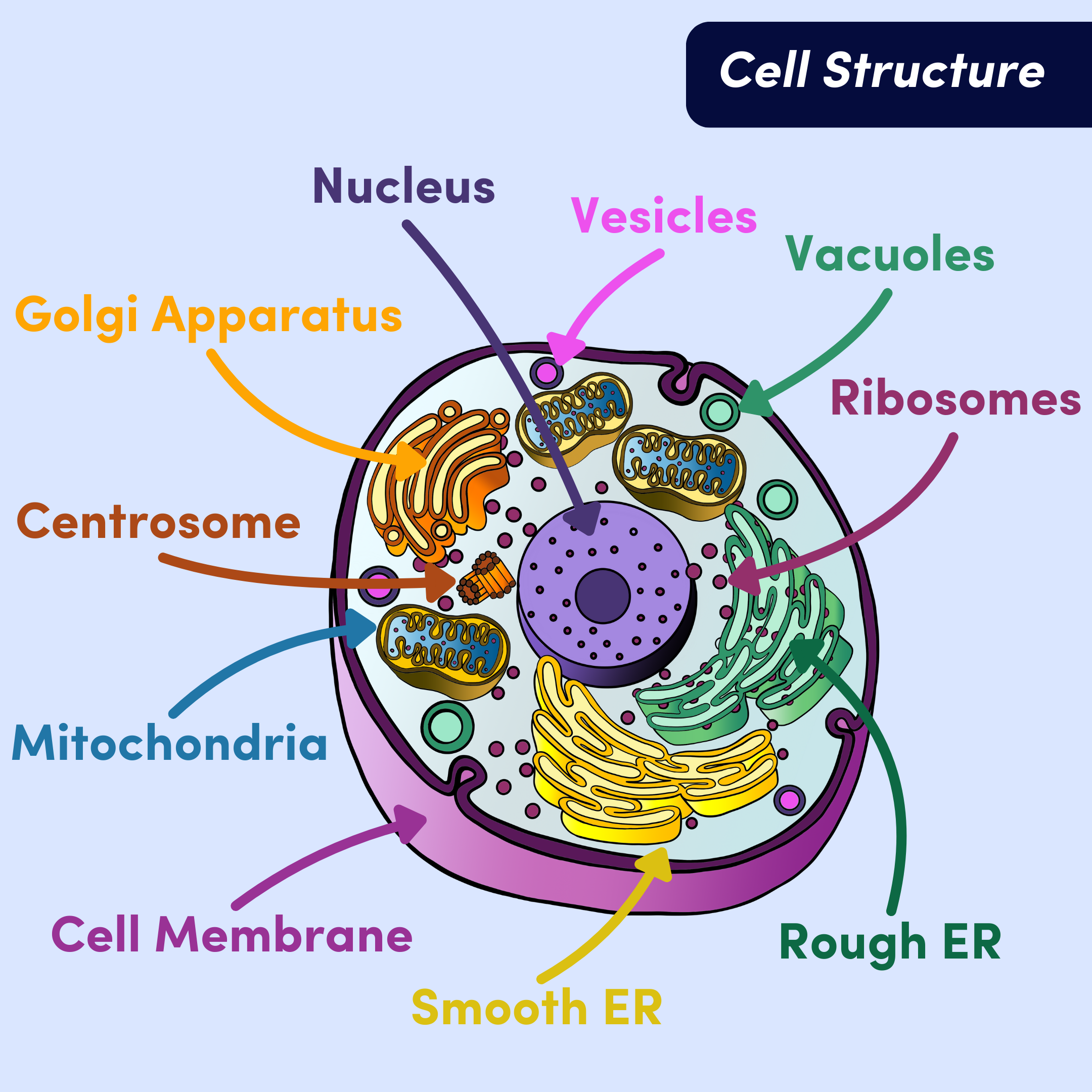
Figure 1. Eukaryotic cell structure and organelles.
Remember: organelles are subcellular structures that have specific jobs within the cell. There are a few main organelles in eukaryotic cells you should know about. Each plays a different role in keeping our cells and, consequently, our bodies, functioning:
Nucleus - this is the largest organelle and holds the cell’s DNA. The nucleus’ function is to regulate gene expression, telling the cell what proteins to make and when.
Endoplasmic Reticulum (ER) - the ER synthesizes and transports lipids, steroids, and proteins. The rough ER is responsible for protein synthesis. The rough ER gets its name because it’s coated in ribosomes (they make proteins). Together, they make proteins that will be mostly transported out of the cell. The smooth ER, on the other hand, is responsible for lipid and steroid production. It is not studded with ribosomes, thus it looks smooth. Every cell has a smooth ER, but the amount varies. The smooth ER helps with detoxification, so liver cells for example, have far more smooth ER than other cells to help clear toxins out of the body.
Golgi Apparatus - the Golgi receives proteins for processing and helps them get to their final destination. Sometimes that means it ships proteins to the membrane to be transported out of the cell, and other times it means it directs proteins to specific organelles within the cell.
Cell Membrane - sometimes called the plasma membrane, the cell membrane protects the cell from the environment outside the cell. It determines what is let in and out of the cell.
Vesicles and Vacuoles - these are both sac-like organelles that serve as taxis for materials around the cell. They store and transport materials to be taken from one organelle to another, and are sometimes used as “biological flask” for reactions to take place. The only difference is that vesicles are smaller than vacuoles.
Lysosomes and peroxisomes are specific vesicles that break down foreign matter and poisons, respectively.
Centrosome - made up of two tiny tubes called centrioles that hang out in the nucleus. Centrioles make sure chromosomes are organized before cell division. This is to avoid mistakes during cell division.
Mitochondria - the main function of the mitochondria is to make energy available to the cell. The mitochondria generates most of the adenosine triphosphate (ATP) during cellular respiration by having a hand in the citric acid (TCA) cycle, electron transport chain (ETC), and chemiosmosis (don’t worry about these yet– we’ll dig into the details momentarily). Essentially, the mitochondria produces energy for the rest of the cell to use to perform work—this is where the “powerhouse of the cell” analogy comes from.
Structurally, the mitochondria has four compartments: the inner membrane, the outer membrane, the intramembranous space, and the matrix. The outer membrane allows ions and small molecules into the intramembranous space. The intramembranous space hosts protons to be used in oxidative phosphorylation. The inner membrane is where the electron transport chain and ATP synthase sit. The matrix is where pyruvate oxidation and TCA cycle take place. More specifics on these functions later, but right now note that these structures foster ATP production. [1]
Together, these organelles work together to keep your cells functioning properly. It’s worth noting that although plant, animal, and fungal cells are all eukaryotic, they have slightly different organelles. Some are common to all three, like the mitochondria and a membrane-bound nucleus (the defining characteristic of a eukaryotic cell), but there is differentiation as well. For example, plant cells have chloroplasts (organelles that help with photosynthesis) while animal cells do not.
While each organelle is important, the mitochondria is fundamental because the energy it produces is necessary for all of the other organelles to function. Mitochondria are therefore fundamental to metabolism, and a major interest here at Calorify.
Mitochondria are distinct from other organelles because they have their own DNA. It is thought that at some point, multiple cells joined together to form eukaryotic cells. Starting as a symbiotic relationship where one cell was living inside another, they eventually spent so much time together and worked so closely that they evolved together. Today, one cannot live without the other. Evidence to support this theory includes the fact that mitochondria have their own DNA, and that this DNA more closely resembles that of a prokaryote [2]. There are several copies of mitochondrial DNA (mtDNA) per mitochondria, and it’s circular just like the DNA found in prokaryotes.
Because mitochondria are so fundamental to energy metabolism (and ATP cannot be stored) each cell can have anywhere from 1,000-2,500 mitochondria [3]. But, it also varies from cell to cell. In heart muscle cells, mitochondria take up 20-30% of the cell’s volume, but comparatively, up to 67% of skeletal muscle might be occupied by mitochondria. Or on the other end of the spectrum, red blood cells don’t have mitochondria at all [4]. Mitochondria are cell specific in that way.
**
Metabolism is the sum of all chemical reactions occurring within a cell that maintain life. These reactions keep you alive and thinking, healing, digesting, playing, growing, and more. On the most simple level, your metabolic rate is how much oxygen you turn into carbon dioxide (i.e., cellular respiration).
There are 4 main steps to cellular respiration:
Glycolysis,
Pyruvate oxidation,
The citric acid cycle (TCA cycle), and
Oxidative phosphorylation (OXPHOS).
Glycolysis, pyruvate oxidation, and TCA cycle make NADH and FADH2 which carry electrons to the electron transport chain. The electron transport chain (ETC) pumps protons (H+) across the inner mitochondrial membrane, “fueling” ATP synthase which makes the most ATP out of any of the steps (30-32 ATP). Together, the ETC and ATP synthase perform OXPHOS, where the ETC oxidizes NADH and FADH2 and ATP synthase phosphorylates ADP. We will explore oxidative phosphorylation in more depth shortly.
Figure 2. Cellular Respiration. Glycolysis is the first step of cellular respiration. It turns glucose into pyruvate, NADH, and ATP. The pyruvate is then used in pyruvate oxidation to create Acetyl CoA, NADH, and CO2. Acetyl CoA is then fed into the citric acid cycle (TCA cycle) where NADH, FADH2, ATP, and CO2 are produced. All of the NADH and FADH2 that were produced thus far are then fed into the ETC (orange). The ETC uses electrons from NADH and FADH2 to pump protons across the inner mitochondrial membrane (this is the oxidation piece). ATP synthase (purple) uses the protons to phosphorylate ADP, making ATP (this is the phosphorylation piece).
Dynamics
Now let’s chat about mitochondrial function. We’ve reviewed that it’s key for energy production, but how? And what else is the mitochondria responsible for?
Mitochondria make the most ATP through oxidative phosphorylation (OXPHOS). OXPHOS describes the process of the Electron Transport Chain (ETC) and ATP synthase working together to make ATP (and water). Electrons enter the ETC via NADH and FADH2 (which, just note that they are electron carriers) that are produced earlier on in the cellular respiration process. The ETC pumps protons (H+) across the inner mitochondrial membrane to create a proton gradient (more protons and more charge on one side) between the intramembranous space and the matrix. This gradient provides protons for ATP synthase to use and actually make ATP. ATP synthase takes ADP (adenosine diphosphate) and uses protons to add a phosphate group to it to make ATP (adenosine triphosphate).
Figure 3. Cellular respiration with breakdown of OXPHOS. Orange numbered bubbles indicate cellular respiration steps, with OXPHOS being broken into two parts, oxidation and phosphorylation. Frames: A) Zoomed in view of where cellular respiration takes place and the mitochondrial components involved. B) The first two steps of cellular respiration: (1) glycolysis, which takes place in the cytosol, and produces pyruvate to be used in (2) pyruvate oxidation, which produces acetyl CoA– an input for (3) TCA cycle. C) TCA cycle produces NADH and FADH2. D) The NADH and FADH2 are used by the ETC to move protons into the intramembranous space. This is (4.1), the oxidation piece of oxidative phosphorylation. E) A proton gradient is formed with the higher concentration of protons in the intramembranous space, and the lower concentration in the matrix. F) ATP synthase takes advantage of this gradient and uses the intramembranous protons to phosphorylate ADP, making (30-32) ATP. This is (4.2), the phosphorylation piece of oxidative phosphorylation.
Potential energy is stored in that new phosphate bond that, when broken, can be used to perform work. As ATP gets transported around the cell, the ADP-phosphate bond is broken, providing energy for other cellular work. However, ATP cannot be stored long-term, so it’s often used very quickly within cells. ATP is recycled in the cell via the adenine nucleotide translocator (ANT) which sits in the inner mitochondrial membrane [5]. ANT grabs ADP that has been floating in the cytosol and swaps it with ATP from the matrix, distributing energy out to the cell, and bringing ADP back to be “recharged”.
But how is this regulated? How does the cell know when it needs more energy and when it can slow down? When there isn’t ADP readily available in the mitochondrial matrix, it means that ATP isn’t being used up rapidly because ADP isn’t being “returned” to be “recharged”. When ADP is added to the matrix, however, it is quickly turned into ATP by the ATP synthase proton channel. Remember: a proton gradient exists across the intramembranous space and the matrix (and is key to ATP synthase function). As ATP is being produced rapidly, the electrochemical gradient is reduced, signaling to the cell it needs to recruit more stored fats and carbs to supply more protons to keep moving these reactions forward [5]. In other words, when your cells aren’t burning many calories, they aren’t receiving chemical signals to produce more ATP.
Calories are simply a unit that represent energy stored in the chemical bonds in our food (and that’s why you see Calories on food labels—see our blog post on Calories on food labels for more information on this topic). Cells can either use those calories to perform work or they can be stored as fat tissue for later use. The calories being burned to perform your daily functions (work, exercise, recovery, etc.) is what Calorify’s DLW test measures: total daily energy expenditure. Calorify also measures total energy intake: analyzing your weight changes to see if you’ve “stored” extra calories. Caloric burn is highly individual and depends on how your mitochondria convert food into energy and how you use that energy.
Part of what makes your caloric burn so unique is how efficient your mitochondria are. Your mitochondrial efficiency is driven by what’s called the coupling efficiency of OXPHOS—how efficiently you turn dietary calories (energy from food) into ATP (energy cells can use). If protons are pumped across the inner membrane efficiently, you get the “best bang for your buck” and get the most ATP for the least amount of heat for each calorie used. This is an example of tightly coupled mitochondria. The opposite, loosely coupled mitochondria, would require more calories and produce more heat to make smaller amounts of ATP.
**
Interestingly, coupling efficiency is partially how warm blooded animals maintain a stable body temperature. Their mitochondria do more work when they need to heat up (think about shivering, the extra movement burns more calories!) and they burn less when they’re warm enough [5]. So, another function of the mitochondria is to produce heat.
**
Another component of efficiency is mitochondrial fusion and fission. “Particularly, mitochondrial fusion is positively associated with increased energy efficiency and ATP production, while a shift towards mitochondrial fission results in a drop in mitochondrial efficiency and a concomitant increase in [reactive oxygen species (ROS)] production.” [6]
Unfortunately, OXPHOS produces ROS and, even more unfortunate, the mitochondria doesn’t have much protection against them. ROS are bad because they are incredibly reactive, causing damage to the cell’s DNA, lipids, and proteins, ultimately making the cell function improperly. OXPHOS takes place in the inner mitochondrial membrane, exposing mitochondrial DNA to ROS, making them susceptible to mutation [6]. mtDNA have a particularly high mutation rate, and this is probably due to their chronic exposure to ROS [5]. Oftentimes if mitochondria mutate or experience damage, they will self-destruct, however, that’s not always the case, and that’s when we start to see mitochondrial diseases.
Dysfunction and Disease
Mitochondrial dysfunction is used to describe cases where mitochondria aren’t working fully or properly compared to a healthy state. San Millan argues that “decreased mitochondrial capacity” or “mitochondrial impairment” could be more appropriate terms in many cases [source]. In a healthy state,
Because mitochondria are so essential to so much of our life function, there are major impacts when dysfunctional. The United Mitochondrial Disease Foundation states, “Because mitochondria perform so many different functions in different tissues, there are literally hundreds of different mitochondrial diseases… Because of the complex interplay between the hundreds of genes and cells that must cooperate to keep our metabolic machinery running smoothly, it is a hallmark of mitochondrial diseases that identical mtDNA mutations may not produce identical diseases.” [7].
Symptoms vary widely and can include loss of muscle coordination and weakness, problems with hearing/vision, learning disabilities, heart/liver/kidney disease, gastrointestinal problems, and neurological problems, including dementia. Other conditions that might involve mitochondrial dysfunction include Parkinson’s disease [7], Alzheimer’s disease [8], Bipolar disorder [9], Schizophrenia [10, 11, 12], Chronic Fatigue Syndrome [13], Huntington’s disease [14], and aging [15, 16].
In addition to all of the pathologies above, cancer, obesity, and type 2 diabetes are also thought to be related to mitochondrial dysfunction. These conditions are all related to inflammation, which is thought to be tightly coupled to mitochondrial dysfunction. Hotamisligil 2006 poses ‘metaflammation’ as metabolically induced inflammation, which is triggered by caloric surplus and looks similar to classic inflammation [17]. Abnormal cytokine production is one of the hallmarks of inflammation. Cytokines are chemical signals (usually proteins) that trigger inflammation and notify the immune system to do its job [17]. This chronic inflammation can damage healthy cells.
The balance between metabolism and inflammation is delicate. Their interconnectedness was evolutionary advantageous in certain conditions, allowing organisms to redistribute energy based on certain immune responses. But, chronic imbalance can be detrimental. For example, tumor necrosis factor-⍺ (TNF-⍺) is a proinflammatory cytokine that works closely with insulin inhibitors. Experiments have been done in mice and humans to show that TNF-⍺ is overexpressed in adipose tissue (fat tissue). When TNF-⍺ was added (simulating overexpression), it caused insulin resistance, but when removed, insulin sensitivity improved [17]. This shows that there is a clear link between TNF-⍺, diabetes, obesity, and chronic inflammation, but it’s hard to pin down the degree to which these pathologies are due to TNF-⍺ alone. Inflammatory signals (like cytokines) work closely in networks, so it’s hard to say how much any individual signal contributes to diabetes or obesity. However, it is clear that they have an effect.
Outside of specific cytokines, the general inflammatory response associated with adipose tissue and other metabolically important sites, such as the liver, might be involved in disease progression. This is probably because adipose tissue and the liver are organized such that they both are close to immune cells, allowing continuous interactions between metabolism and inflammation [17]. “Of note, obesity and visceral fat accumulation in particular are underlain by a low-grade chronic inflammation (Hotamisligil, 2006) and increased ectopic fat storage in metabolically active tissues including skeletal muscle and liver, a phenomenon termed lipotoxicity (Unger, 2002).” [6]
Now that we’ve established that inflammation is related to diabetes and obesity, what causes such chronic inflammation? Calorie excess. Calorie excess can be caused by eating too much or not exercising enough.
In the literature, calorie excess has been demonstrated to cause a host of issues but most notably, increased ROS production. Remember that ROS are produced by mitochondria as a by-product of OXPHOS, the process by which ATP is produced. “When calorie intake is in excess or the capacity of oxidative phosphorylation is limited, the electron transport is impaired in the electron transport chain and has a higher chance of being converted to ROS. These ROS can damage proteins, lipids and mtDNA of mitochondria and further increase the production of ROS.” [2] The highest rate of ROS production happens when calorie excess is high, and energy expenditure is low. Together, they influence the proton gradient and ETC such that “excess electrons are transferred to oxygen without ATP production.” [2].
What is the practical result of this? Cellular damage, which contributes to further mitochondrial dysfunction. This makes skeletal muscle and the heart work less efficiently. In other words, it speeds up aging [2]. “Clinical manifestations that have been linked to mtDNA mutations affect the brain, heart, skeletal muscle, kidney, and endocrine system, the same tissues affected in aging. Specific symptoms include forms of blindness, deafness, movement disorders, dementias, cardiovascular disease, muscle weakness, renal dysfunction, and endocrine disorders including diabetes.” [5].
Additionally, it has been shown that mutated mitochondria and cells with mutated mitochondria tend to be preferentially cloned. It is thought that this is probably an attempt to compensate for less efficient mitochondria. However, as the amount of mutant mtDNA increases, output declines, tissue function declines, ROS increases, and cells die faster. This accumulation of less effective mitochondria is aging [5]. Symptoms appear when the healthy cells can no longer maintain function [5]. Chronic oxidative stress (from ROS) causes mitochondria to decline until they can no longer function. At this point, cell death is activated. With age, the rate at which this happens increases, and symptoms of age related diseases pop up [5].
Aging is also generally related to a decline in insulin sensitivity, which is responsible for keeping glucose in check by promoting glucose uptake in skeletal muscle and suppressing glucose production in the liver [4]. Excess glucose can cause issues like plaque build up in blood vessels and weight gain—which both have their own complications. Insulin resistance is associated with having less mitochondria and abnormal mitochondria, leading to lower ATP synthesis [4]. The age-related decline in insulin sensitivity is associated with reduced OXPHOS and increased fat accumulation.
In the case of insulin sensitivity and obesity, the main protein that drives mitochondrial fusion (which is beneficial for better mitochondrial efficiency) is downregulated in people with Type II Diabetes Mellitus (T2DM) and obesity [6]. However, studies have found that when these people lost weight, these protein levels were restored. This suggests that calorie/nutrient excess drives mitochondrial dynamics, and also that abnormalities or “damage” are reversible [6]. T2DM was found to be responsible for a 60% decrease in insulin-stimulated glucose uptake, 80% increase in intramyocellular fat, and 30% decrease in mitochondrial OXPHOS in insulin resistant subjects [2]. Additionally, chronic mitochondrial oxidative stress can explain all of the symptoms/features of T2DM [5]. This suggests that mitochondrial dysfunction could be an important mechanism of insulin resistance. Several recent studies have pointed out that it’s hard to say whether mitochondrial dysfunction causes insulin resistance or if the reverse is true [2], however, “boosting mitochondrial function remains a promising strategy to improve insulin sensitivity” [6].
Promoting Health
There are two main treatments on the horizon that hope to promote mitochondria health, and I’m sure you’ve heard them before: exercise and diet.
Exercise
Exercise is probably the single greatest thing you can do for your mitochondria. Exercise, specifically aerobic and endurance exercise, directly affects your mitochondria. It affects mitochondrial biogenesis, mitochondrial fusion and fission, and mitochondrial autophagy. All of these directly impact cardiac health, and can help prevent cardiovascular disease. Exercise effectively makes your mitochondria more efficient. It reduces the amount of mitochondrial fission and improves autophagy/biogenesis. Exercise “cleans up” what you don’t need and repairs what you do.
“Exercise also improves insulin action and glucose tolerance in insulin-resistant subjects and animal models. Substantial evidence indicates that aerobic exercise stimulates mitochondrial biogenesis by stimulating gene expression” of biogenesis relevant genes. “Endurance exercise training increases mitochondrial size, number, and oxidative activity contributing to improved whole-body glucose metabolism. Increased expression of eNOS by physical activity may stimulate mitochondrial biogenesis. Moderate-intensity physical activity combined with weight loss improves insulin sensitivity through increasing skeletal muscle electron transport chain activity and increasing mitochondrial cristae (without changing mtDNA content). Age-associated reduction in expression of mitochondrial genes and mitochondrial biogenesis is restored with aerobic exercise.” [4].
While exercise isn’t additive to your burn, moving your body does make you burn more calories. Remember that calorie excess drives mitochondrial dysfunction. Movement burns calories such that damaging feedback cycles in your body don’t have enough fuel. This has been demonstrated in Pontzer 2018, where activity level was investigated in relation to immune system, HPA axis, and reproductive system activity. Pontzer showed that physical activity promotes health (but you can also overdo it! - although that isn’t as much of a concern for the general population) [18].
Additionally, San-Millan 2018 compares metabolic biomarkers during exercise between athletes, average active people, and people with metabolic syndrome [19]. Such biomarkers like lactate and fat oxidation rate can tell us more about how efficiently your mitochondria are working. Through these proxy measurements, San-Millan shows that the athletes are more metabolically fit, more flexible. Their mitochondria are better able to handle situations that would otherwise lead to dysregulation (additionally, his 2023 paper [20] shows that during detraining, mitochondrial activity decreases dramatically). This work shows that exercise is key to keeping your mitochondria flexible and ready for anything that might get thrown their way!
Diet
There are two components to diet, what you eat and how much you eat. Both components have an effect on mitochondria.
Protein. There is no shortage of protein studies in both humans and animals. On the whole, studies seem to show that protein-rich diets lead to increased muscle protein synthesis, release of particular gut hormones and insulin, and eating less overall. Physiologically, after a protein heavy meal, your blood has more protein in it, particularly branched chain amino acids (leucine, isoleucine, and valine), which have been shown to increase mitochondrial biogenesis and function in both humans and mice. A practical effect of this is better cardiac and skeletal muscle function [6]. “This is plausible because amino acids are used as precursors of tricarboxylic acid cycle intermediates and produce approximately 10–15% of total metabolic energy in animals apart from serving as a metabolic fuel during exercise.” [6].
Because of this positive impact of branched chain amino acids, some have postulated that branched chain amino acid supplements could promote mitochondrial heath by inducing mitochondrial biogenesis [6]. Additionally, a high-protein diet has been shown to have physiological advantages when compared to a high-carbohydrate diet like increased fatty acid oxidation (using fat as energy by drawing from fat stores) [6]. However, more research is needed to more fully understand how amino acids play a role in mitochondrial function [6].
Other Foods to Seek Out. There are many reasons why a food might be good for you, but one reason is if it contains bioactive compounds. Bioactive compounds don’t have much nutritional value in the traditional sense, but they are present in small quantities in some foods and have great health benefits.
Coenzyme Q10 or Ubiquinone is abundant in animal products and key to the ETC. It is a potent antioxidant, meaning it protects cells from oxidative damage [6].
Of note, individuals with T2DM have been observed to have a coenzyme Q10 deficiency. Supplementation has been posed as a way to improve glycaemic control via a direct effect on mitochondrial function (Shen and Pierce, 2015).
Quercetin is a polyphenol that is in a group of flavonoids (flavonoids get their name from the Latin word flavus, meaning yellow, which is their color in nature). Quercetin is abundant in apples, onions, peppers, berries and leafy greens. It has been widely researched and shown to have a positive effect on skeletal muscle and mitochondrial biogenesis (in rats, so more work is needed to confirm this in humans) [6].
Resveratrol is a stilbenoid polyphenol found in grapes, nuts, and berries. While there has been interest in it for its potential to increase longevity, it’s more recently emerged for its connection to metabolic health. It seems as though it is able to modulate mitochondrial function, biogenesis, and oxidative metabolism. In rats it has been shown to counteract the negative effects of a high fat diet. In theory it helps with insulin sensitivity, but in practice its effect probably isn’t great enough to affect mitochondrial function to the degree of increasing insulin sensitivity (but if exercise is added, the effect is greater) [6].
Other honorable mentions include epicatechin (another flavonoid), coumestrol, and omega 3 polyunsaturated fatty acids (PUFAs). Administration of epicatechin rich cocoa to T2DM individuals resulted in better mitochondrial biogenesis in skeletal muscle. Coumestrol, which is found in legumes, has also been shown to increase mitochondria biogenesis in cultured muscle cells. And omega 3 PUFAs are known to be anti-inflammatory and tend to increase mitochondrial fusion (as opposed to long-chain saturated fatty acids which cause inflammation) [6].
Overall, food can help promote mitochondrial function by scavenging ROS and protecting mitochondria from oxidative damage. More studies are needed to show the full impact of these food bioactive derivatives and other compounds on mitochondria.
Calorie Restriction. We know that an oversupply of calories can increase ROS production and cause inflammation. Calorie restriction works in the opposite way and improves insulin sensitivity and delays metabolic and age-related diseases. The thought here is that calorie restriction effectively slows the “rate of living” or the rate of aging on the cellular level by reduced oxidative damage [6]. “In a systematic review of over 157 studies on the effects of CR on mitochondrial ROS production, Walsh et al. reported that 46% of studies report a reduction in ROS production in muscle, and 60% of studies detected a reduction in mitochondrial ROS production in brain (Walsh et al., 2014). Of note, significant reduction in ROS production was more likely if the duration of the caloric restriction exceeded 20 months. Discrepancies in outcomes between studies could be due to differences in the tissue examined, the duration of CR, the degree of energy restriction, the dietary fat load or source imposed.” [6]. Studies done in humans have also been highly variable, warranting further research. Additionally, intermittent fasting needs more research in order to reveal impacts on mitochondrial dynamics.
Pharmacological treatments are also being assessed for where diet and exercise aren’t feasible. The first type of treatment is to promote mitochondrial biogenesis (creation), and the second is to reduce ROS production—both of which aim to increase mitochondrial function [4]. Pharmacological targets that stimulate mitochondrial biogenesis have already been developed in part, including drugs like thiazolidinediones (insulin sensitizers). Similarly, there are products that reduce ROS production like metformin, angiotensin receptor blockers, and antioxidants [4].
Ultimately, a systems chemistry approach will probably be best (there is no stand alone magic pill). Mitochondrial dysfunction is complex and it doesn’t look the same in every case. Treatments will likely focus on creating a ‘new homeostasis’ modifying more than one target [17].
Conclusion
At the beginning of this article, we learned that the mitochondria has a very important role in the cell–to produce energy to be used by other organelles in the cell (this is where the “powerhouse of the cell” analogy comes from). We then learned how ATP is produced via OXPHOS and reviewed other dynamics that contribute to mitochondrial impact on the cell, such as ROS impacts, fusion and fission. Finally, we explored how the body is affected by mitochondrial dysfunction, how it relates to diabetes, obesity, and other pathologies, and how proper diet and exercise can help protect mitochondrial function.
So, mitochondria are a key interest at Calorify, because we’re measuring all of the work that they do! Mitochondrial function and energy use continues to be an increasingly important factor in health and disease as we unravel its role in some of the most prevalent diseases of the century–cancer, obesity, and more. Calorify’s doubly labeled water measurement is driving our understanding of the impacts of mitochondria function on the whole body forward, and we’re excited to be a part of such important work.
Have more questions about mitochondria or suggestions for future mini-series like these? Reach out to Calorify’s Research Scientist, Erica Svendahl via support@calorify.com.
References
[1] Kwak et al. 2010. Mitochondrial metabolism and diabetes. Journal of Diabetes Investigation.
[2] Martin et al. 2010. The Origin of Mitochondria. Scitable by Nature Education.
[4] Kim et al. 2008. Role of Mitochondrial Dysfunction in Insulin Resistance. Circulation Research.
[15] Srivastava. 2017. The Mitochondrial Basis of Aging and Age-Related Disorders. Genes.
[17] Hotamisligil. 2006. Inflammation and metabolic disorders. Nature.
[18] Pontzer. 2018. Energy Constraint as a Novel Mechanism Linking Exercise and Health.
[20] San-Millán. 2023. The Key ROle of Mitochondrial Function in Health and Disease.